When discussing the chemical bonding in transition metal oxides, scandium oxide (Sc2O3) often comes under scrutiny due to its unique position as an early transition metal oxide. The nature of the bond—whether it is ionic or covalent—depends on a variety of factors, including the elements involved, their electronegativities, and the overall chemical environment. This article aims to dissect these factors in the context of scandium oxide and provide a clearer understanding of its bonding nature.
The Nature of Chemical Bonds: A Brief Overview
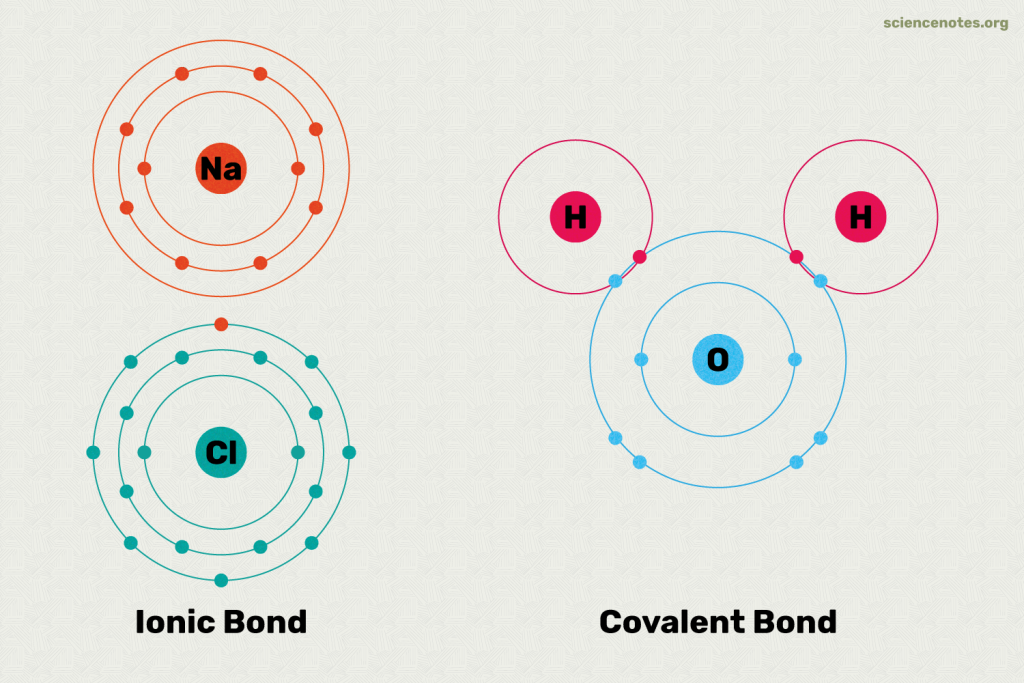
Chemical bonds are the forces that hold atoms together in compounds. They can be broadly categorized into two types: ionic and covalent. Ionic bonding occurs when electrons are transferred from one atom to another, leading to the formation of positively and negatively charged ions.
This type of bonding is typically observed when a metal reacts with a nonmetal. Covalent bonding, on the other hand, involves the sharing of electron pairs between atoms. This is more common among nonmetals.
Scandium Oxide: Ionic with Covalent Character
Scandium oxide (Sc2O3) is formed between scandium, a transition metal, and oxygen, a nonmetal with a high electronegativity. The significant difference in electronegativity between scandium and oxygen suggests an ionic bond due to the transfer of electrons to form Sc^3+ and O^2− ions. However, the distinction between ionic and covalent bonds is not always black and white, especially in compounds involving transition metals like scandium.
Transition metals are known for their ability to form complex bonds due to the d-orbitals’ involvement in bonding. While scandium oxide is primarily ionic, it also exhibits some covalent character. This is due to the partial sharing of electrons that occurs alongside the electron transfer, a phenomenon that is not uncommon in transition metal oxides.
Transition Metal Oxides: A Spectrum of Bonding
Transition metal oxides present a wide range of bonding characteristics, from highly ionic to significantly covalent. This variability is attributed to several factors:
Electronegativity: The greater the difference in electronegativity between the metal and oxygen, the more ionic the bond is likely to be. Since scandium has a relatively low electronegativity compared to oxygen, the resulting oxide is more ionic.
Oxidation States: Transition metals can adopt multiple oxidation states, influencing the nature of the bonding. Higher oxidation states can lead to more covalent character due to the involvement of more electrons in bonding.
D-Orbital Participation: The availability and involvement of d-orbitals in bonding can enhance the covalent character. Scandium, being an early transition metal with a relatively simple electron configuration ([Ar]3d^14s^2), has limited d-orbital participation, thus favoring ionic bonding.
Why Scandium Oxide Stands Out
Scandium oxide is often highlighted for its bonding nature due to scandium’s position as the first element in the transition metals series. Its electron configuration limits extensive covalent interactions with oxygen, leading to a predominantly ionic oxide. However, it is a misconception to state that “only scandium oxide is covalent” among transition metal oxides. Many transition metal oxides exhibit a blend of ionic and covalent characters, with the balance tipping based on the specific metal and its chemical context.
The Spectrum of Bonding in Transition Metal Oxides
The transition from scandium oxide’s ionic nature to the more covalent character of oxides formed with later transition metals exemplifies the complexity of chemical bonding within this group. Factors such as electronegativity, electron configuration, and oxidation state play crucial roles in determining the bonding nature. As one moves across the transition series, the increase in d-orbital electrons and the subsequent ability for more complex bonding interactions result in a gradual shift towards more covalent character in the metal oxides.
Conclusion
In summary, scandium oxide (Sc2O3) is primarily ionic due to the significant electronegativity difference between scandium and oxygen, coupled with scandium’s limited ability for extensive d-orbital covalent bonding.
However, like many transition metal oxides, it exhibits some degree of covalent character, challenging the binary classification of bonds as purely ionic or covalent. The nature of bonding in transition metal oxides spans a spectrum, influenced by a complex interplay of chemical properties and behaviors. Understanding this spectrum requires a nuanced appreciation of the factors at play, highlighting the fascinating complexity of chemical bonding.
To inquire about high-quality scandium alloys, please contact us.
